4.2 Density of Liquids
Density of Regular & Irregular Objects
- Some liquids are thinner, and some are thicker, so they have different densities.
- Their densities can be calculated in the laboratory through experiments.
- To calculate the density, measure the mass of the empty measuring cylinder/beaker using a top-pan balance.

- Add some amount of liquid to the measuring cylinder and record its volume from the scale marked on it.
- Record the mass of the empty cylinder with the help of a top-pan balance to the nearest gram, say m1.
- Measure the total mass of the cylinder + liquid mass, say mass m2.
- The following relationship can calculate the mass m of the liquid:
- The following relationship can calculate the mass m of the liquid
m = m2 – m1
- The density of the liquid can be calculated by using the following formula.
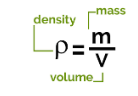
Q: The diagram shows an experiment to find the density of a liquid. What is the density of the liquid?
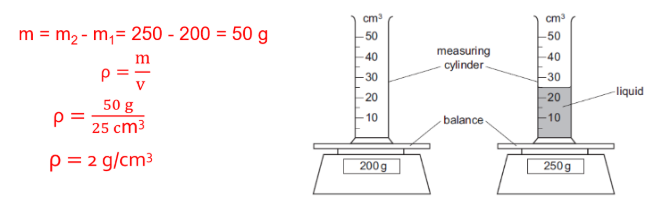
Density of an Irregular Shaped Solid
- Measure the volume V1 of a certain amount of liquid by using a measuring cylinder.
- Tie the solid object by a fine thread and dip it gently into the liquid, and measure volume V2 of the liquid plus the volume of the solid object.
Volume of solid, (V) = V2-V1
- Measure the mass “m” of the solid object by using a top-pan balance to get density.
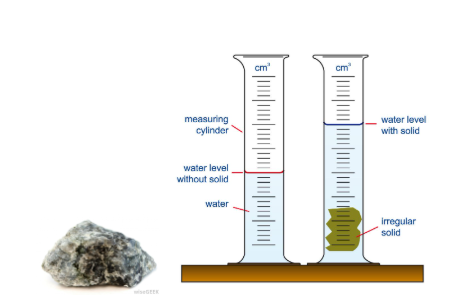
Q: A student wants to calculate the density of an irregular piece of metal having a mass of 44 g
The following figures show the volume of water in both measuring cylinders. Calculate the density of the metal.
V = V2 – V1 = 50 – 40 = 10 cm3